R&D
For energy utopia with you
Hydrogen Energy
Hydrogen energy refers to the energy generated by using hydrogen as a fuel. The hydrogen consumed in a fuel cell reacts with oxygen to produce only water, without emitting greenhouse gases, making it a clean fuel that has gained global attention
As an energy carrier, hydrogen stores and transports energy produced from other resources, and it is utilized in various industries such as vehicles, power plants, and energy storage system (ESS). Hydrogen produced from renewable energy sources will play a crucial role in the future hydrogen economy.

Hydrogen is categorized as green, blue, or gray depending on the production method. In particular, green hydrogen is produced using renewable energy sources such as solar and wind power.
As a sustainable energy source with zero CO₂ emissions, green hydrogen plays an important role in achieving carbon neutrality and RE100 goals by contributing to decarbonization across various industrial sectors.
What is the electrolysis?
Water electrolysis is an electrochemical redox reaction that uses electrical energy to split water into hydrogen and oxygen.
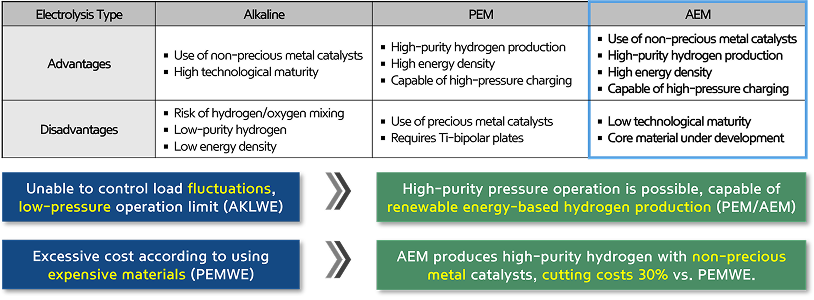
The process is categorized into Alkaline, PEM, and AEM electrolyzers depending on the type of electrolyte membrane used, with AEM offering advantages in cost efficiency and high-purity hydrogen production.
AEMWE
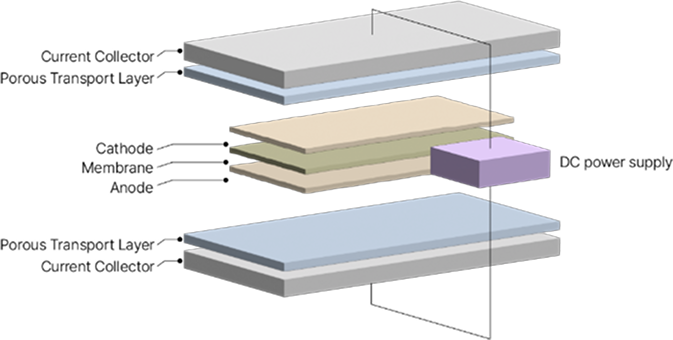
Anion Exchange Membrane Water Electrolysis (AEMWE) is a technology that produces hydrogen and oxygen by electrolyzing water in an alkaline environment.
This technology utilizes an anion exchange membrane to transport hydroxide ions (OH⁻) between the electrodes.
During water electrolysis, oxygen is produced at the anode, while hydrogen evolved at the cathode. AEM hydrogen production systems offer cost advantages over other electrolysis systems by using inexpensive non-precious metal catalysts.
AEMWE exhibits high efficiency and competitiveness in hydrogen production when combined with renewable energy sources.
- Anode: 4OH⁻ → 2H₂O + O₂ + 4e⁻
- Cathode: 4H₂O + 4e⁻ → 2H₂ + 4OH⁻
Optimal for
-
Dynamic Response
-
Low Cost
-
Non-PGM
-
High Pressure
-
High Performance
-
High Purity








